Prof. Víctor Muñoz
Victor Muñoz obtained his PhD in Biophysical Chemistry in 1995 in the group of L. Serrano at UAM (Madrid, Spain) and EMBL (Heidelberg, Germany). In 1996 he joined the group of William A. Eaton as postdoctoral fellow at the National Institutes of Health (Bethesda, Maryland, USA). By 2000 he became Assistant Professor at the University of Maryland where he started his independent career as PI. In 2005 he was named Tenured Associated Professor in this institution. In 2007 he came back to Madrid as Professor of Centro de Investigaciones Biológicas (CSIC). In 2009 he was named Adjunct Professor of the University of Maryland. By April 2013 he moved his group to Centro Nacional de Biotecnología and IMDEA Nanosci- ence (where he is Associate Researcher). He is Ad hoc reviewer for both scientific journals and granting agencies. Throughout his career he has been received honors and awards like Camille and Henry Dreyfus New Faculty Award (2000), Packard Fellowship for Science and Engineering (2001), Searle Scholar (2002), Marie Curie Excellence Grant (2007), EMBO Member (2009) and ERC Advanced Grant (2013).
Research Lines
Our group investigates the conformational-functional behavior of proteins to answer questions concerning the intimate behavior of proteins using a multidisciplinary approach that combines physical chemistry, advanced molecular spectroscopy (ultrafast and single-molecule), structural biology, computer science, protein engineering, and molecular biology. Our efforts during last years have centered on three major areas:
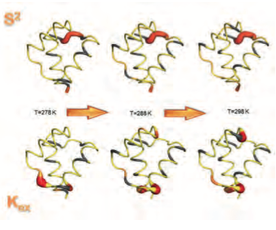
- Experimental and theoretical analysis of protein folding ensembles, where we have developed a catalogue of folding archetypes corresponding to small single-domain proteins with elementary combinations of secondary structure elements.
- Investigation of the molecular rheostat hypothesis, proteins able to produce analogue signals at the singlemolecule level rather than the binary outputs of conventional molecular switches. These rheostats could offer a new variety of nanobiotechnological applications. In parallel, we analyze the biological roles of conformational rheostats in coordinating protein networks via conformational selection, in the phe- nomenon of DNA sliding and homing- to-target, and as molecular springs in macromolecular assemblies.
- Engineering of macromolecular assemblies, where we are engineering macromolecular devices from monomeric globular proteins. This effort borrows ideas from molecular evolution to implement a design strategy that would facilitate domain swap- ping between otherwise monomeric proteins by engineering their folding behavior reducing their intrinsic folding cooperativity.